Our Science Has Been Verified By 26 Research Studies & 40 Patents
All studies have been conducted in the USA and at top research facilities. All studies use FDA validated standard measurement indices.
If you’d like to see more of our 26+ studies, please contact us and we’ll be happy to provide.
Ready for Some Geek-Talk?
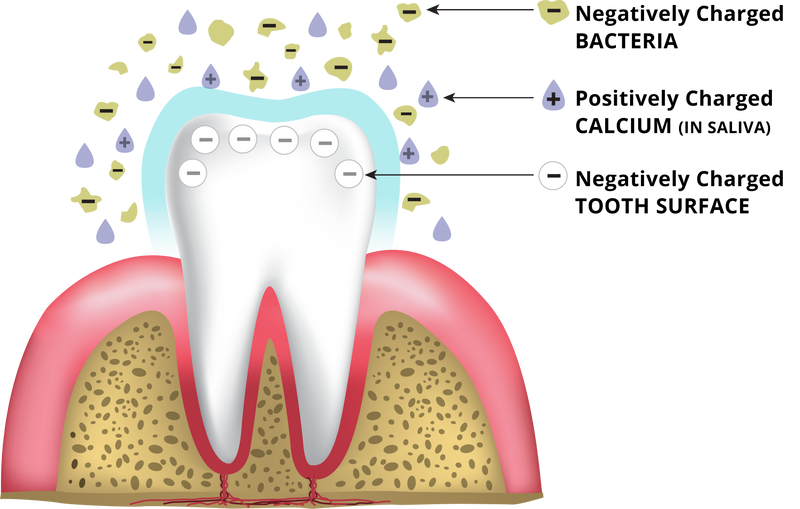
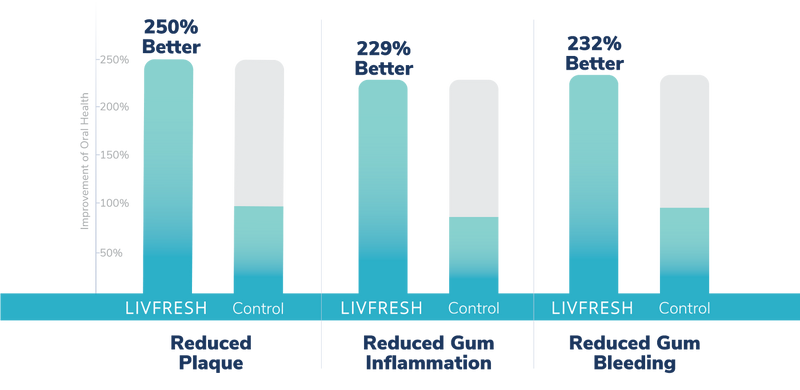
How do you know LIVFRESH is superior to other toothpastes?
Study Summary
As compared to the control anti-gingivitis toothpaste, LIVFRESH users improved plaque levels by more than 250%, gingival inflammation improved by more than 229%, and gum bleeding improved by more than 232%.
Study References
1. Multimodality imaging of the effects of a novel dentifrice on oral biofilm. Ajdaharian J. et al, Lasers Surg Med 2014 Sep;46(7):546-52. doi: 10.1002/lsm.22265, PMID: 24916419
2. Effects of a Novel Dental Gel on Plaque and Gingivitis: A Comparative Study. Dadkhah M, et al. Dentistry 4:239, 2014 Jun 1;4(6):239. doi: 10.4172/2161-1122.1000239, PMID: 26052472
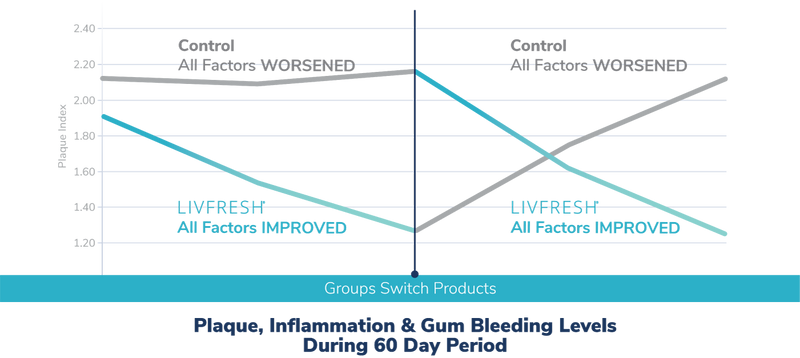
What happens if you stop using LIVFRESH?
Study Summary
According to the ADA, gum disease is caused by plaque. This study showed:
- 100% of subjects starting on the control toothpaste showed reduced plaque when they switched to LIVFRESH
- 100% of subjects starting on LIVFRESH showed increased plaque when they switched to the control toothpaste
Study References
1. Plaque Removal and Gingival Health after Use of a Novel Dental Gel: A Clinical Study. Nayudu, Anuradha et al. Dentistry (Sunnyvale, Calif.) vol. 6,10 (2016): 396. PMID: 28286702, doi:10.4172/2161-1122.1000396.
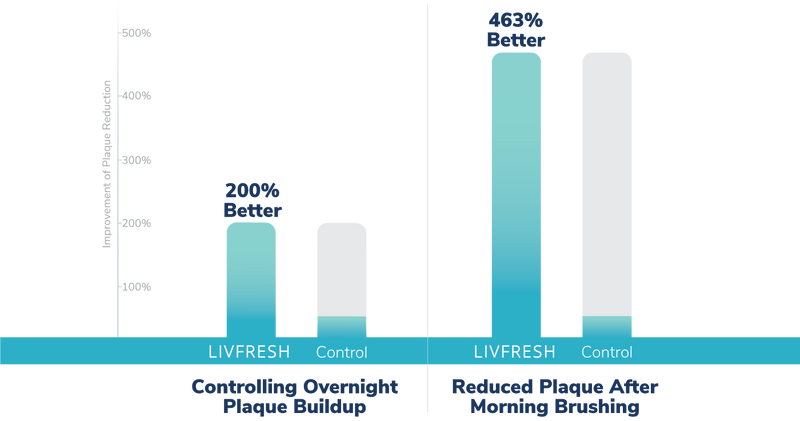
Does LIVFRESH prevent overnight plaque buildup?
Study Summary
In this double-blind study, LIVFRESH was found to be 2 times better than the leading toothpaste at controlling overnight buildup on teeth after a single brushing at night. After users brushed with LIVFRESH in the morning, the dental gel was found to be more than 4 times more effective at reducing plaque than a popular toothpaste.
Study References
1. Dental Plaque Removal and Re-Accumulation: A Clinical Randomized Pilot Study Evaluating a Gel Dentifrice Containing 2.6% Edathamil. Anbarani, AG, et al. The Journal of Clinical Dentistry vol. 29,2 (2018): 40-44. PMID: 30211989
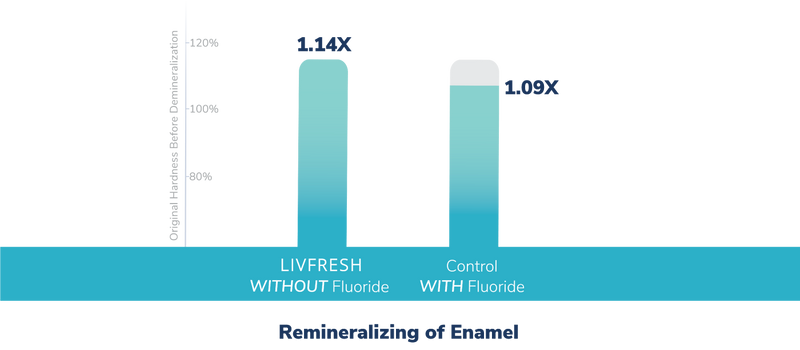
Does LIVFRESH remineralize dental enamel?
Study Summary
This double-blind study used scanning electron microscopy to evaluate dental enamel health of brushing with LIVFRESH vs. standard fluoride toothpaste. No difference was found in enamel health.
Additionally, as shown in the graph, brushing with LIVFRESH dental gel without fluoride remineralized enamel similar to a commercially available standard fluoride toothpaste.
Study References
1. A Double-Blinded, Randomized Study Evaluating the In Vivo Effects of a Novel Dental Gel on Enamel Surface Microstructure and Microhardness.
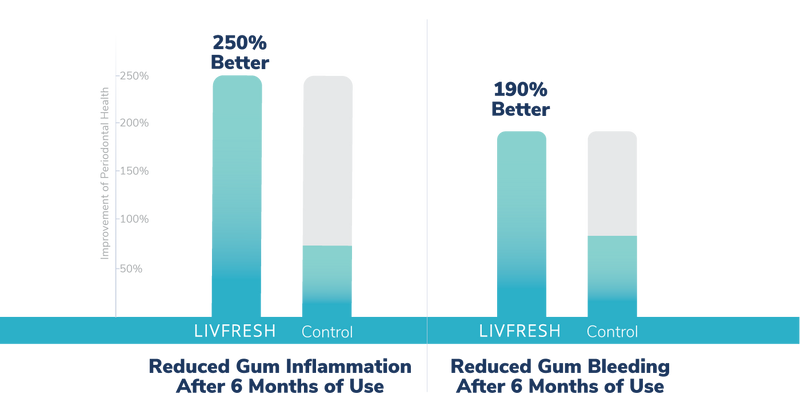
What happens if patients with periodontitis brush with LIVFRESH for 6 months?
Study Summary
Results showed that over a 6-month period, as compared to a market leading anti-gingivitis toothpaste, twice a day brushing with LIVFRESH showed significant improvements in periodontal pocket depths, gingival inflammation, gum bleeding, and plaque.
As shown in the figure below, as compared to the control stannous fluoride toothpaste, the LIVFRESH group showed 80% of the diseased pockets depths improved, and very large relative improvements in gum inflammation, and gum bleeding over a 6-month period.
Study References
1. Evaluating efficacy of a novel dentifrice in reducing probing depths in Stage I and II periodontitis maintenance patients: A randomized, double-blind, positive controlled clinical trial. Kaur, Maninder et al. Journal of Periodontology vol. 92,9 (2021): 1286-1294. doi:10.1002/JPER.20-0721. DOI: 10.1002/JPER.20-0721. PMID: 3333104